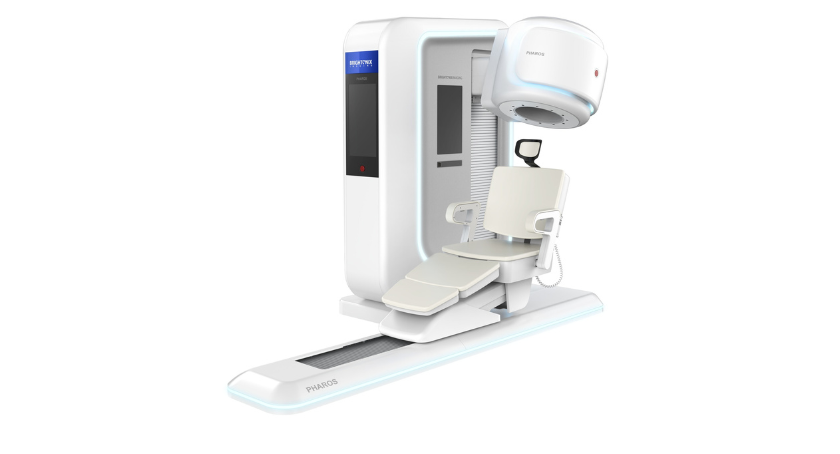
The US Food and Drug Administration has clarified the Brightonix Imaging flagship, the Pharos PET scanner, a state-of-the-art clinical positron emission tomography system (PET), which offers health service providers a powerful instrument for recognizing diseases, precise diagnosis and optimized treatment planning.
Pharos is multifunctional with the ability to physically orient the patient seat and detector configurations for the extremity and breast imaging as well as converting them into the lying and seated modes for the imaging of the brain.
The most important functions and advantages of the PET scanner pharos include:
- Unprecedented image clearance: With the help of advanced detector technology, the pharos-pet scanner provides high-resolution images, so that clinicists can visualize even the most subtle anomalies for precise diagnosis and treatment.
- Compact footprint: The pharos scanner was developed with a space-efficient footprint, which makes installation easier in a variety of clinical environments. Its optimized design offers greater flexibility for hospitals and imaging centers with a limited space without affecting the imaging performance.
- Flexible applications: Its multifunctionality enables tailor -made configurations for brain, breast and extremities -pet scans and at the same time offers a more comfortable setup that improves the entire patient experience.
- User -friendly interface: With an intuitive interface and an optimized workflow, the PET scanner of pharos of clinicians and technologists is easy to improve the user experience and to ensure seamless integration into medical practices.
“The FDA Clearance for the Pharos PET scanner is a monumental performance for us and for the entire medical imaging community. This technology will enable the instruments for the detection and treatment of degenerative diseases of the neuro -degenerative diseases and with greater prerequisites,” said Brightonix Imaging foundation.
The development of Pharos was supported by the Korea Medical Development Fund (KMDF), which reflects a joint effort in several government agencies in order to promote innovative medical imaging technologies.
After Brightonix Imaging has secured the FDA approval, he will now introduce the Pharos PET scanner of US medical facilities.
The Brightonix imaging was founded with a commitment to innovation and excellence in medical imaging and develops state-of-the-art solutions that improve diagnostic accuracy, patient care and the clinical performance of the entire general performance. The company's portfolio includes advanced clinical PET, medical artificial intellilance software and preclinical PET/CT image -like systems.